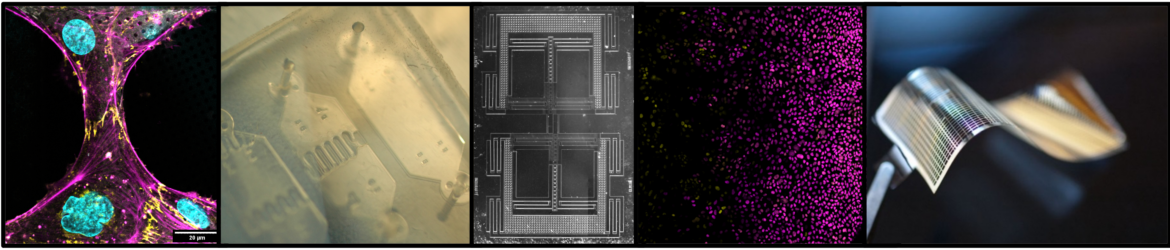
I conducted my graduate research with Dr. Yosi Shacham (Physical Electronics) and Dr. Slava Krylov (Mechanical Engineering) at Tel Aviv University. The main goal of my research was to develop MEMS based on electroactive polymers, smart materials also known as “artificial muscles”. I was initially drawn to the field of polymer MEMS due to its many applications for improving human life such as tools for less invasive surgery, active prosthetics, and lab-on-a-chip devices. A major challenge to the development of electroactive polymer MEMS lies in the integration of smart materials with microsystems. I developed fabrication processes for working with a range of electroactive polymers at the micro and nano-scales and microfabricated and characterized novel polymer MEMS. This work contributed to the ”toolkit” of electroactive polymer actuator technologies by innovating new device architectures and providing a scientific basis for investigating the electromechanical properties of electroactive polymers in MEMS. My graduate research was recognized by several awards including a prestigious award from the American Vacuum Society granted to one graduate student each year.
In addition, I had the opportunity to work on several international collaborations that influenced my research direction and made interdisciplinary collaboration a hallmark of my academic style. In the European Union Heart-e-Gel Consortium, I characterized the electromechanical response of a novel biocompatible hydrogel developed for operation in cardiovascular systems [1,2]. Next, I collaborated with Dr. Gregory Washington’s group at UC Irvine to develop electroactive polymer actuators with integrated mechanical sensing [3]. Following a grant from the Sackler Fund for Convergence Research in Biomedical, Physical, and Engineering Sciences, I integrated electroactive polymers with microfluidic circuitry as a visiting student researcher in Dr. Liwei Lin’s lab at UC Berkeley [4]. At Tel Aviv University, I collaborated with Dr. Tal Dvir’s group to develop a freestanding cardiac patch for on-line monitoring and regulation of tissue function, which resulted in a patent and a publication in Nature Materials [5]. My role in this project was to contribute my MEMS expertise to fabricate a flexible microelectrode array compatible with cardiac cell culture. Upon observing the spontaneous contraction of cardiac cells, I realized that cells themselves are fascinating micro-machines with exquisite sensing and actuating capabilities! This collaboration strongly influenced my research direction toward applying the engineering methodologies I had mastered to the study of cell behavior. I resolved to immerse in quantitative biology for her postdoctoral research.
I was awarded a ChEM-H Mechanobiology Postdoctoral Fellowship to train with Drs. Beth Pruitt, Alex Dunn, and Bill Weis at Stanford University through their ongoing collaboration to study the molecular basis of how mechanical force affects cell-cell junctions. These labs created an ideal research environment for me to leverage my engineering background toward developing in vitro assays to study mechanobiology, while acquiring hands-on training in established biological research methodologies. In the Pruitt lab, I contributed to the design of a silicon MEMS device for applying shear perturbation to epithelial monolayers [6]. Appreciating my enthusiasm for bio-microfabrication, Dr. Pruitt presented me with the challenge of controlling cell shape and position on delicate cryo-ET supports. In collaboration with the Hanein lab, I applied maskless photopatterning to innovate a robust micropatterning technology that enables high-resolution mechanobiology studies by cryo-ET [7], a method that is rapidly gaining traction in the field. I was in awe of the power of cryo-ET to enable visualization of the preserved, internal structure of the cell—the most complex machine known to man—at unprecedented resolution. This emerging microscopy field presented the perfect opportunity for me to contribute my engineering expertise toward understanding cellular function. In the Dunn lab, I tailored my micropatterning technology to study the architecture of the intercellular junctions that connect endothelial cells and quantified the ability of this technique to increase the rate of cryo-ET data collection [8]. These studies deepened my understanding of cell mechanics by considering cellular processes at the molecular level. In parallel, I established a productive collaboration with Dr. Kyle Loh’s lab, applying microfluidics to questions in developmental biology [9,10].
References:
[1] L. Engel, O. Berkh, K. Adesanya, J. Shklovsky, P. Dubruel, S. Krylov, Y. Shacham-Diamand.Actuation of a novel Pluronic-based hydrogel: Electromechanical response and the role of applied current. Sensors and Actuators B: Chemical 191, 650-658. (2013) [2] N. Jackson, P. Verbrugghe, D. Cuypers, K. Adesanya, L. Engel, et al. A Cardiovascular Occlusion Method Based on the use of a Smart Hydrogel. IEEE Transactions on Biomedical Engineering 62 (20), 399-406. (2014) [3] L. Engel*, K.R. Van Volkinburg*, M. Ben-David, G.N. Washington, S. Krylov, Y. Shacham-
Diamand (*equal contribution). Fabrication of a self-sensing electroactive polymer bimorph actuator based on polyvinylidene fluoride and its electrostrictive terpolymer. Proc. SPIE 9798, Electroactive Polymer Actuators and Devices (EAPAD), 2016, Las Vegas, Nevada, USA. [4] L. Engel, C. Liu, N. Hemed, Y. Khan, A. Arias, S. Krylov, Y. Shacham-Diamand, L. Lin. Local electrochemical control of hydrogel microactuators in microfluidics. J Micromech Microeng 28 (10), 105005. (2018) [5] R. Feiner, L. Engel, S. Fleisher, M. Malki, A. Shapira, Y. Shacham-Diamand, T. Dvir. Engineered hybrid cardiac patches with multifunctional electronics. Nature Materials. (2016) DOI:10.1038/NMAT4590 [6] M. Garcia, E. Sadeghipour, L. Engel, W.J. Nelson, B.L. Pruitt. MEMS Device for Applying Shear and Tension to an Epithelium combined with Fluorescent Live Cell Imaging. J Micromech Microeng 30 125004. (2020) [7] L. Engel, G. Gaietta, L.P. Dow, M.F. Swift, G. Pardon, N. Volkmann, W.I. Weis, D. Hanein, B.L. Pruitt. Extracellular matrix micropatterning technology for whole cell cryogenic electron microscopy studies. J Micromech Microeng 29 (11). (2019) [8] L. Engel*, C.G. Vasquez*, E.A. Montabana, B.M. Sow, M.P. Walkiewicz, W.I. Weis, A.R. Dunn (*equal contribution). Lattice micropatterning for cryo-electron tomography studies of cell-cell contacts. Journal of Structural Biology 213(4), 107791. (2021) [9] K.W. Cui*, L. Engel*, C.E. Dundes, T.C. Nguyen, K.M. Loh, A.R. Dunn (*equal contribution). Spatially controlled stem cell differentiation via morphogen gradients: A comparison of static and dynamic microfluidic platforms. Journal of Vacuum Science & Technology A 38 (033205). (2020) doi: 10.1116/1.5142012 [10] L. Engel*, K.W. Cui*, K.J. Liu, V.T. Vachharajani, C.E. Dundes, S.L. Zheng, M. Begur, K.M. Loh, L. Ang, A.R. Dunn (*equal contribution). A microfluidic platform for anterior-posterior human endoderm patterning via countervailing morphogen gradients in vitro. under review in iScience.